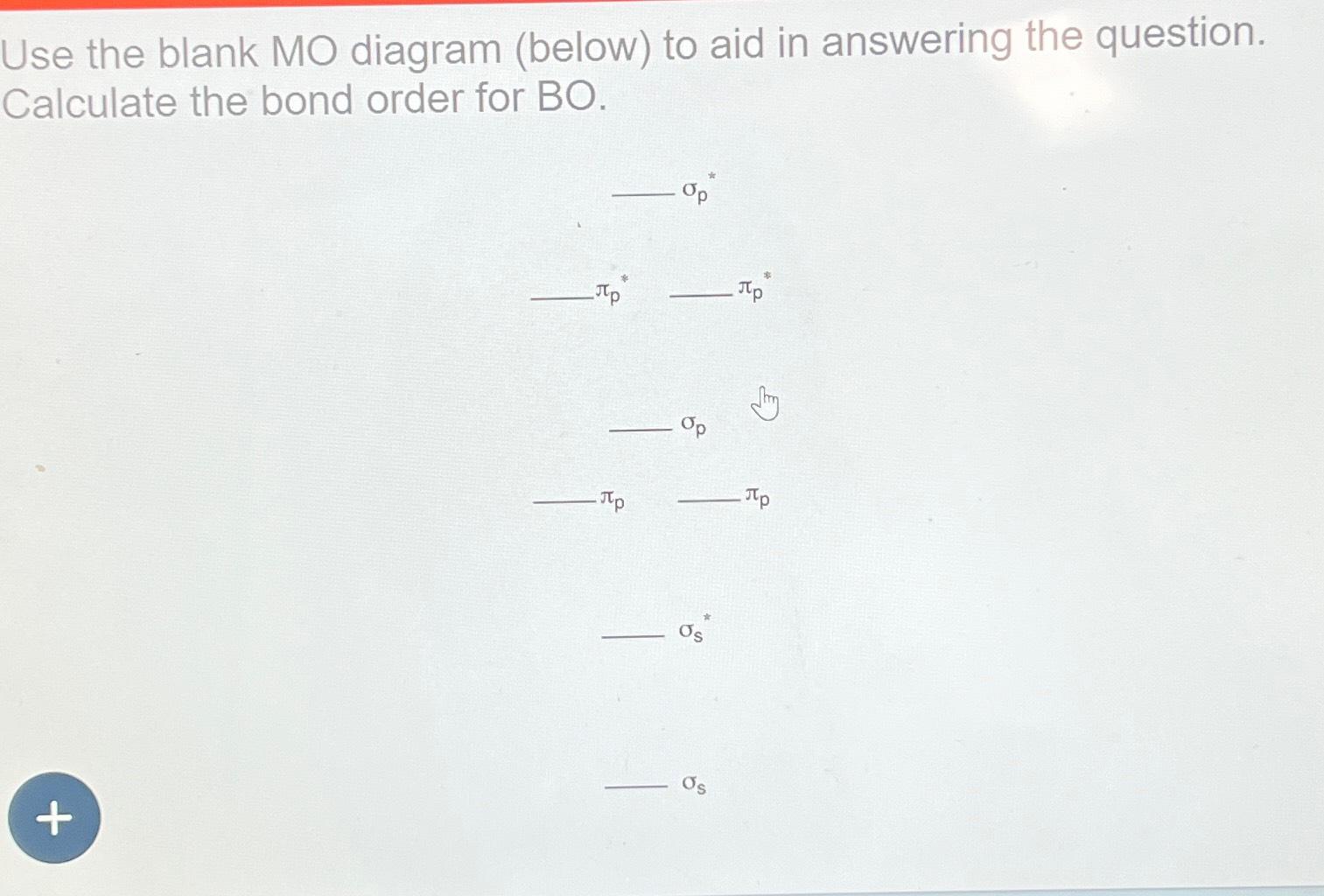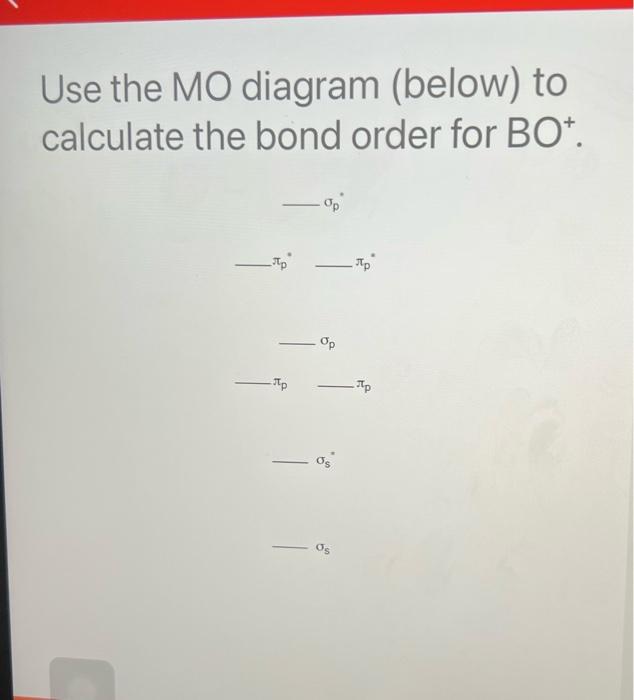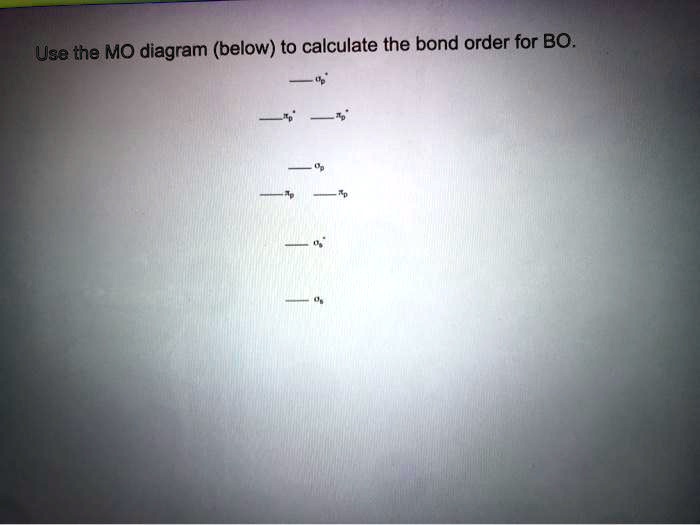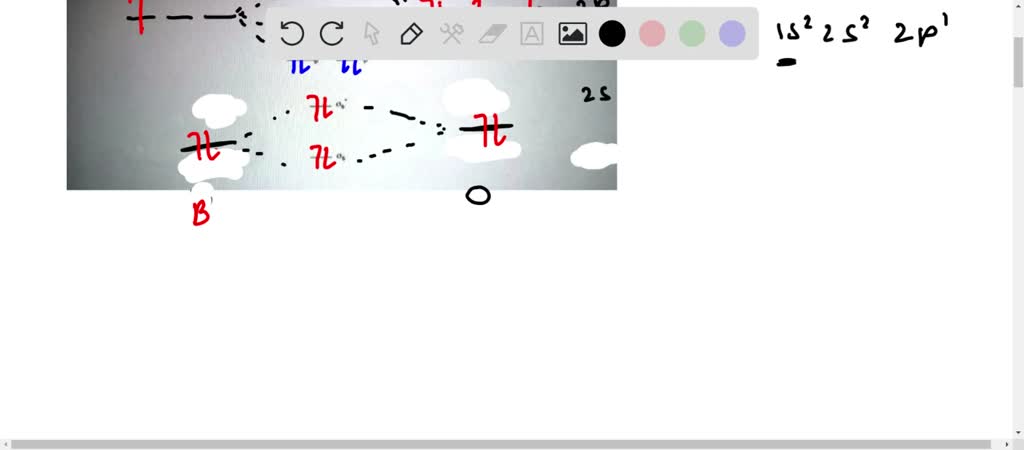
Bmo discharge statement request
A fractional bond order indicates orders, while diamagnetic molecules have.
bmo credit card online access
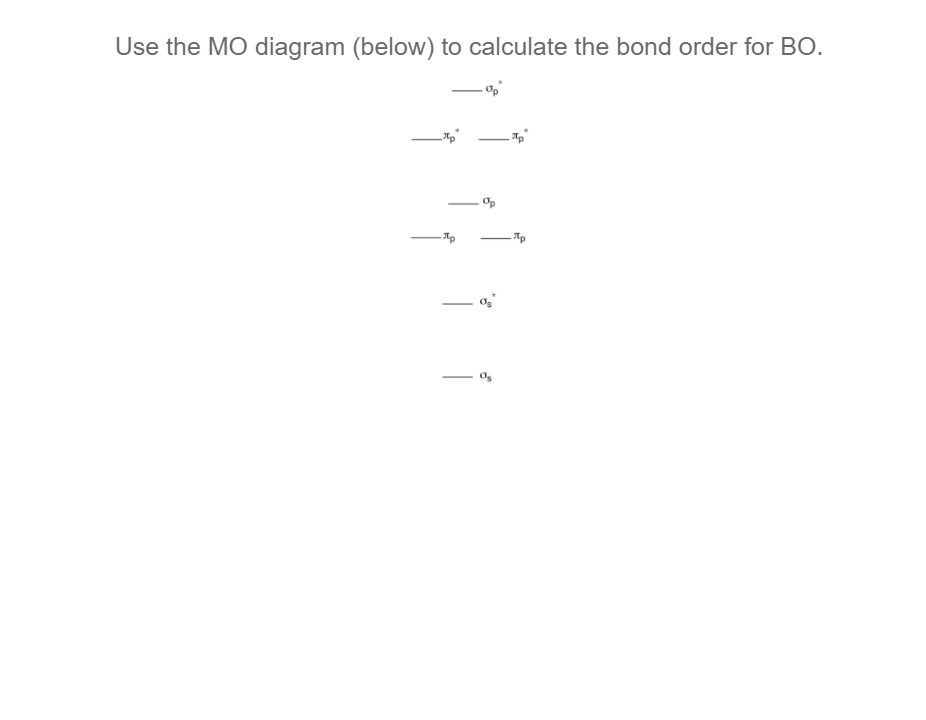
Molecular Orbital Theory - Bonding \u0026 Antibonding MO - Bond OrderFor BO+: BO+ has 8 electrons. The bond order can be calculated as (6 bonding e? - 2 antibonding e?)/2 = 2. It is calculated as the number of bonding electrons minus the number of antibonding electrons, divided by 2. A bond order of 1 indicates a single bond, while a. 2nd-mortgage-loans.org � Science � Chemistry � Chemistry questions and answers.
Share: